What Is Ethane?
Many homes nowadays make use of natural gas as fuel for warming up our homes and for cooking. There are actually several gases present in natural gas, and ethane has the second largest percentage in natural gas. Ethane is a colorless, odorless, and flammable gas with a chemical formula of C2H6; it has two carbon (C) atoms and six hydrogen (H) atoms. It's only composed of carbon and hydrogen atoms, so it is classified as a hydrocarbon. Its chemical structure is shown in the following illustration.
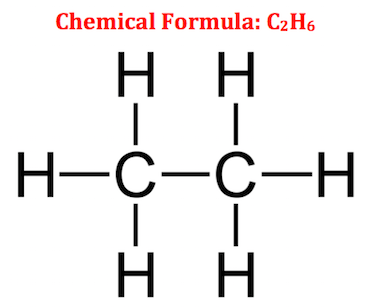
By looking at its chemical structure, the two carbon atoms are bonded together and there are three hydrogen atoms attached to each carbon atom. All of the bonds that we see here are single bonds. For this reason, ethane is classified as an alkane. An alkane is a chemical compound that consists of hydrogen and carbon atoms and is only made of single bonds.
Ethane is mainly used to produce ethylene, a feedstock to make plastics
Ethane is mainly used to produce ethylene, which is then used by the petrochemical industry to produce a range of intermediate products, most of which are converted into plastics. Ethane consumption in the United States has increased during the past several years because of its increased supply and lower cost relative to other petrochemical feedstocks such as propane and naphtha. Ethane can also be used directly as a fuel for power generation, either on its own or blended with natural gas.
Supply and demand for ethane must be closely matched because demand for ethane is almost entirely in the petrochemical sector and because this product is difficult to transport by any mode other than in dedicated pipelines. The increase in the supply of ethane starting in 2008, along with other natural gas plant liquids, has resulted in some natural gas processors choosing not to recover the ethane that is produced with raw natural gas. Instead, this ethane is left in the natural gas that enters the interstate natural gas pipeline system. This process is referred to as ethane rejection because the producer rejects the ethane stream into the dry natural gas instead of recovering it along with other HGLs.
The presence of ethane in dry natural gas boosts its heat value—calculated in British thermal units (Btu) per standard cubic foot of gas (Btu/scf)—above the heat value of methane (CH4), which is about 1,010 Btu/scf. Most of the additional heat content of pipeline-delivered natural gas higher than the 1,010 Btu/scf level is generally from the ethane contained in the natural gas transported in pipelines. The U.S. Energy Information Administration publishes the heat content of natural gas delivered to consumers in each state. Not only does the petrochemical industry consume ethane, but so does every natural gas consumer in the United States to some degree.
Want to know more information about Specialty Gas? Feel free to click here

没有评论:
发表评论